X chromosome inactivation is a fascinating genetic mechanism that plays a crucial role in balancing gene expression between males and females. In humans, females possess two X chromosomes while males have just one, leading to the need for females to deactivate one of their X chromosomes to prevent an overabundance of gene products. This intricate process, involving silencing through a unique chromosomal structure, has been a significant focus of research, notably by Jeannie T. Lee’s lab at Harvard Medical School. Their recent studies have shed light on the underlying mechanisms of X chromosome inactivation, which may open doors to potential treatments for genetic disorders like Fragile X Syndrome and Rett Syndrome. Understanding how this critical process works not only enhances our knowledge of chromosomal function but also highlights promising therapeutic strategies for alleviating the burdens of these debilitating conditions.
The phenomenon known as X chromosome silencing refers to the selective inactivation of one X chromosome in female cells, ensuring equal gene dosage between genders. This vital process helps maintain genetic equilibrium, as excess genes from both parents can disrupt normal cellular function. Researchers are delving into the complexities of this mechanism, as it has significant implications for conditions such as Fragile X Syndrome and Rett Syndrome, both linked to mutations on the X chromosome. Recent chromosomal breakthroughs in understanding X chromosome silencing reinforce its importance in the realm of genetic research, offering hope for novel treatment avenues for various chromosomal disorders. Enhancing our comprehension of this process is crucial, as it paves the way for innovative therapeutic solutions aimed at combating the challenges posed by these genetic anomalies.
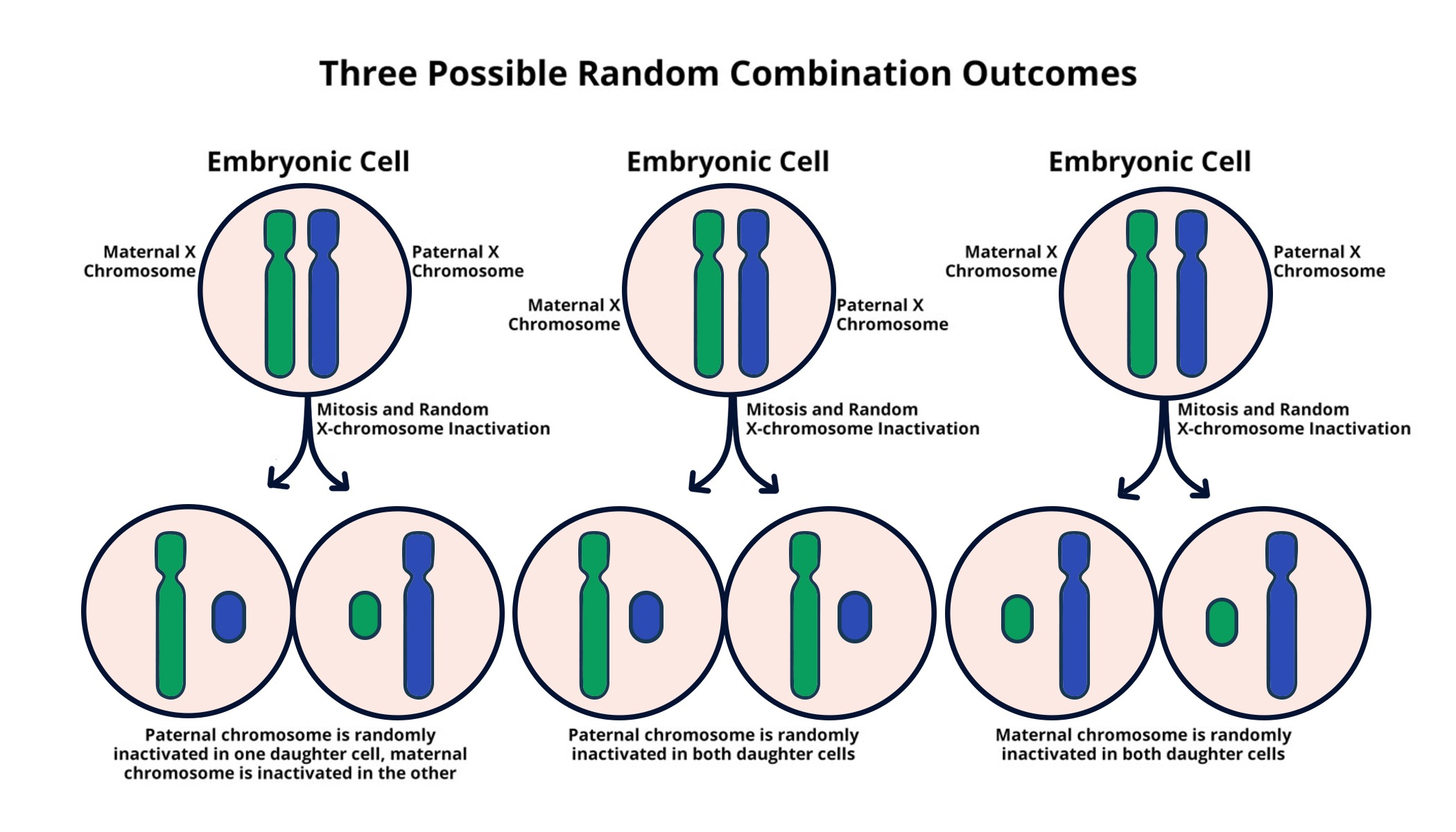
The Mechanism Behind X Chromosome Inactivation
X chromosome inactivation is a crucial biological process that ensures dosage compensation between sexes, particularly in mammals. This process, where one of the two X chromosomes in females is randomly silenced, enables a balance of gene expression from the X chromosomes between genders. The phenomenon is governed primarily by the X-inactive specific transcript (Xist) gene, which plays a pivotal role in orchestrating the silencing mechanism. Understanding this intricate process is fundamental, as it serves as the backbone for developing potential therapies for various genetic disorders linked to the X chromosome.
Recent findings from researchers, including Jeannie Lee at Harvard Medical School, have elucidated how certain molecular interactions contribute to the effectiveness of X chromosome inactivation. The interaction between Xist RNA and a gel-like substance surrounding the chromosomes, referred to as the chromatin matrix, is essential for the successful silencing of the X chromosome. By modifying the physical properties of this Jell-O-like substance, Xist allows other molecules to infiltrate and engage with the X chromosome effectively, leading to its inactivation. This understanding could have profound implications for treating genetic disorders such as Fragile X Syndrome and Rett Syndrome.
Implications of X Chromosome Research on Genetic Disorders
The implications of understanding X chromosome inactivation extend far beyond basic biology; they carry significant potential for therapeutic innovations in treating genetic disorders. Disorders like Fragile X Syndrome and Rett Syndrome, which are primarily caused by mutations on the X chromosome, could benefit immensely from new strategies to reactivate silenced genes. By targeting the inactivated chromosome, researchers aim to restore the function of healthy genes that are otherwise stifled by their mutated counterparts. This restorative process could lead to revolutionary treatments that ameliorate symptoms and enhance the quality of life for affected individuals.
Furthermore, the breakthroughs in Lee’s lab represent an intriguing intersection of genetics and therapeutic intervention. As studies progress, the focus on developing techniques to ‘unsilence’ genes in the X chromosome promises a future where individuals with genetic disorders may find relief through gene therapy approaches. By specifically addressing the unique challenges posed by X-linked disorders, researchers are paving the way for a more nuanced understanding of genetic disease management. This could lead to novel treatments that not only address symptoms but also tackle the root causes of diseases like Fragile X and Rett Syndrome.
Understanding Fragile X Syndrome and Its Genetic Basis
Fragile X Syndrome is one of the most prevalent forms of inherited intellectual disability, predominantly affecting males. This syndrome is caused by a mutation in the FMR1 gene located on the X chromosome, which leads to a deficiency in the production of a critical protein necessary for neuronal function. The relationship between this genetic mutation and the symptoms of Fragile X has been a subject of intense study, as researchers seek to understand how such mutations disrupt normal developmental processes. Effective interventions could hinge on our understanding of X chromosome inactivation and how it impacts gene expression.
Current advances in molecular biology are shedding light on the pathways that contribute to the severity of symptoms associated with Fragile X Syndrome. As researchers explore the possibility of reactivating the silenced genes on the X chromosome, the hope remains that they can restore some level of function lost due to the mutation. By leveraging the insights gained from the study of X chromosome inactivation, scientists aim to design therapies that could significantly improve cognitive and behavioral outcomes for those affected by Fragile X.
Rett Syndrome: A Closer Look at Genetic Mechanisms
Rett Syndrome is another devastating genetic disorder linked to mutations in the X chromosome, primarily affecting females. Characterized by severe cognitive and physical impairments, Rett Syndrome underscores the importance of studying X-linked genes and their regulation. The primary gene implicated in this disorder is MECP2, and its dysfunction leads to a cascade of neurodevelopmental issues. By understanding the X chromosome’s inactivation mechanism, new therapeutic avenues may emerge to mitigate the effects of MECP2 mutations.
Recent research highlights novel strategies derived from insights into X chromosome biology that may rejuvenate the expression of mutated genes like MECP2. Efforts to modulate X chromosome inactivation could provide a dual approach: first, to silence the mutated gene, and second, to restore the normal gene’s function, offering a potential pathway toward treatment. Continuing these lines of investigation provides hope for individuals with Rett Syndrome and their families, aiming for breakthroughs that could one day lead to effective interventions.
The Role of Chromosomal Breakthroughs in Modern Genetics
The realm of genetics is continually evolving, with chromosomal breakthroughs paving the way for revolutionary advancements. Understanding the mechanisms of X chromosome inactivation is one of these breakthroughs, marking a significant chapter in genetic research. Insights gained from the work of Jeannie Lee and her team illuminate previously veiled pathways of gene regulation, shedding light on not only X-linked disorders but also broader biological implications across genetic fields. These insights are invaluable for ongoing innovations in gene therapy, aiming to correct genetic disorders at their source.
Moreover, these developments signify a turning point in how we approach the treatment of chromosomal disorders. By leveraging our understanding of chromosomal structures and functions, researchers are no longer limited to symptomatic treatment but can instead anticipate curative approaches that target the root causes of genetic conditions. The ongoing research into X chromosome inactivation exemplifies the potential these chromosomal breakthroughs hold for changing the future of medicine, emphasizing the importance of interdisciplinary collaboration in achieving meaningful patient outcomes.
Current Research Directions in Genetic Disorders
As research progresses, the focus on genetic disorders continues to deepen, with emphasis on harnessing molecular genetics for therapeutic developments. The exploration of X chromosome inactivation is shifting towards practical applications, especially in conditions like Fragile X Syndrome and Rett Syndrome. Researchers are actively seeking innovative methods to utilize this understanding to develop effective treatments that could alter the course of these genetic disorders. Funding and collaborative efforts, such as those from NIH grants, are accelerating this vital research.
In parallel, the scientific community is observing how advancements in gene editing technologies, like CRISPR, can be integrated with discoveries from X chromosome studies. The combination of chromosomal insights with cutting-edge technology promises to yield powerful new interventions, potentially shifting paradigms in genetic disorder treatment. As the medical landscape evolves, the intersection of genetics and technology will undoubtedly provide novel avenues for diagnosis and management of complex genetic diseases.
Future Perspectives on X Chromosome Therapeutics
The future of X chromosome therapeutics appears promising, with ongoing research illuminating the myriad possibilities for treating X-linked disorders. Scientists are increasingly optimistic about the potential to reactivate silenced genes within the X chromosome, presenting hope for conditions that were once deemed untreatable. As clinical trials for these new methodologies advance, the scientific community is eager to understand the full implications of these treatments for both males and females, especially in light of the differing mechanisms at play.
Continued exploration into X chromosome inactivation not only hints at solutions for Fragile X Syndrome and Rett Syndrome but also raises deeper questions about the nature of gene expression in general. The insights gained from understanding Xist and its interactions with chromosomal structures can reveal fundamental principles of genetics that may apply to a broader range of genetic disorders. The convergence of research in this area is bound to yield therapeutic options with lasting impacts on patient care and genetic disease management.
The Importance of Funding in Genetic Research
Funding plays a crucial role in fostering innovation within genetic research. The studies conducted by Jeannie Lee and her team at Harvard Medical School exemplify the importance of sustained financial support to unravel complex genetic mysteries. Over the years, the National Institutes of Health has provided essential backing for projects focusing on X chromosome inactivation, enabling researchers to delve deeper into the mechanisms governing gene expression. Such support has laid the groundwork for groundbreaking discoveries that have potential therapeutic implications.
As the field of genetics expands, it is paramount that funding continues to flow into areas of high need, such as research on X-linked genetic disorders. The outcomes of these research efforts have a direct impact on the development of treatments that can change lives. With increasing awareness and advocacy for genetic research, there is hope that financial resources will match the growing potential of scientific breakthroughs, accelerating the pace at which we can translate laboratory findings into clinical applications.
Collaboration Across Scientific Disciplines
Collaboration among researchers from varied scientific disciplines is pivotal in advancing the understanding of complex genetic phenomena like X chromosome inactivation. Interdisciplinary teamwork allows for pooling of knowledge and resources, which is especially beneficial in tackling multifaceted challenges presented by X-linked disorders. By combining genetics, molecular biology, and computational methods, researchers can design comprehensive approaches that boost the efficacy of their studies and enhance outcomes for individuals with genetic disorders.
Moreover, such collaborations can stimulate innovative thinking, allowing for the emergence of unique methodologies that transcend traditional study designs. By fostering an environment where experts from various domains share insights and techniques, the scientific community can generate holistic solutions to issues like Fragile X Syndrome and Rett Syndrome. These partnerships not only enhance the potential of research but also accelerate the translation of scientific findings into real-world applications, ultimately contributing to improved therapies for genetic disorders.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in females?
X chromosome inactivation is a crucial biological process that occurs in females, where one of the two X chromosomes is randomly silenced to ensure that the dosage of X-linked genes is balanced with males, who have one X chromosome. This process prevents an overload of gene expression from the X chromosome, thereby maintaining cellular function and preventing diseases related to genetic disorders.
How does X chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is a genetic disorder caused by mutations on the X chromosome. In females with one mutated X chromosome, X chromosome inactivation can render the healthy copy unusable if it is housed within the inactivated X. Understanding X chromosome inactivation can lead to potential treatments by freeing the healthy gene, thereby offering hope for those affected by this disorder.
What role does Xist RNA play in X chromosome inactivation?
Xist RNA is a pivotal component in X chromosome inactivation. It is encoded by a gene on the X chromosome and acts by altering the properties of the surrounding chromosomal structure, facilitating the inactivation. Once engaged, Xist RNA helps enclose the X chromosome in a protective ‘gel-like’ substance, effectively silencing it and preventing gene expression.
Can X chromosome inactivation strategies provide cures for conditions like Rett Syndrome?
Yes, the strategies developed to understand X chromosome inactivation, particularly those that aim to ‘unsilence’ genes, hold promise for treating Rett Syndrome. By activating the healthy version of the gene that may be inactivated, researchers aim to alleviate symptoms associated with this neurodevelopmental disorder.
What are the implications of chromosomal breakthroughs in understanding genetic disorders?
Recent chromosomal breakthroughs in understanding X chromosome inactivation shed light on the mechanisms underlying genetic disorders. These findings not only help elucidate the processes contributing to conditions like Fragile X and Rett Syndromes but also pave the way for developing targeted gene therapies that can address the root causes of these diseases.
How does X chromosome inactivation affect males differently than females?
While males have only one X chromosome and do not undergo X chromosome inactivation, they can still be affected by diseases linked to mutations on the X chromosome. In males, if the solitary X chromosome carries a mutation like that found in Fragile X Syndrome, it can directly impact their health, unlike in females, where the process of inactivation can sometimes mask the effects of such mutations.
What challenges did researchers face in unravelling the mysteries of X chromosome inactivation?
Researchers faced significant challenges in unraveling the complexities of X chromosome inactivation due to its intricate nature and the difficulty in observing these processes in live cells. It took decades of study to understand how Xist RNA interacts with the chromosomal matrix to achieve inactivation, which is now seen as a breakthrough in genetics and cell biology.
| Key Points | Description |
|---|---|
| X Chromosome Complexity | Females have two X chromosomes, while males have one. To avoid excess gene dosage, females inactivate one X chromosome. |
| Mechanism of Inactivation | Inactivation involves a gelatinous substance surrounding chromosomes, allowing Xist RNA to modify its properties and inactivate the X chromosome. |
| Research Contributions | Jeannie Lee’s lab at Harvard has identified how the chromosomal silencing process works, providing insights into potential treatments. |
| Potential Treatments | The research suggests that by unsilencing inactivated X-linked genes, treatments for disorders like Fragile X Syndrome and Rett Syndrome may be developed. |
| Future Directions | Lee plans to continue optimizing strategies for ‘unsilencing’ genes and is preparing for clinical trials. |
Summary
X chromosome inactivation is a crucial process that ensures females have the appropriate dosage of X-linked genes. This genetic mechanism allows for the silencing of one of the two X chromosomes in females, preventing gene overdose. Recent research led by Jeannie T. Lee has uncovered the complexities involved, particularly how the RNA molecule Xist interacts with a gelatinous substance to facilitate this inactivation. The implications of this research are significant, potentially paving the way for innovative treatments for genetic disorders such as Fragile X and Rett syndromes by targeting and restoring the function of mutated genes on the inactivated chromosome.